I’ve been following 24 stem cell stocks for a few years. I haven’t invested in them or any others recently, but rather just find them interesting.
How are these doing in 2023? Mostly not great. In some cases, it is downright disastrous.

Let’s take a quick look at four stocks very much in the news over the last few years that have dropped a lot or even tanked in the past year.
One lesson here is that their ventures into the COVID arena were probably distractions and unwise overall. While some of them experienced a big temporary COVID bump in stock price at the worst of the pandemic, in the long run those efforts may have siphoned off crucial resources and focus. Admittedly, there was some logic for the world to be exploring many options for COVID when things were at their worst, but the rationales for MSC-like or marrow cell approaches were not the strongest.
A tale of 4 stem cell stocks
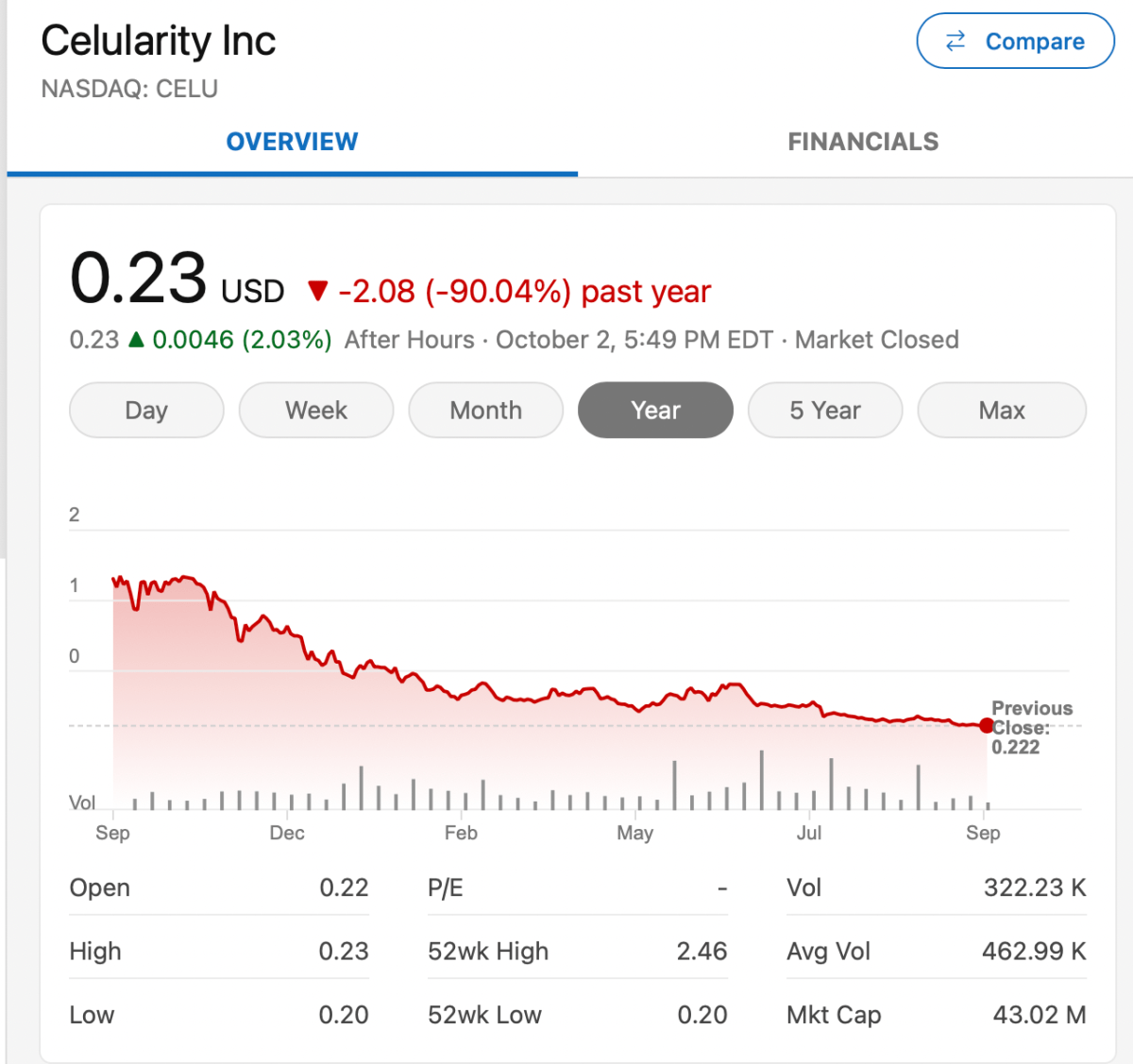
Celularity
Celularity is focused on placenta cell-derived product development.
I believe the company has engaged in some hype over the years. For example, see: Just one word placentas: Can Celularity live up to hype? However, they have some interesting products including NK cells for cancer, which have some potential. I hope they are ultimately successful on the NK effort.
As a side note, I recently raised concerns about Hariri’s venture with Peter Diamandis and Tony Robbins in the anti-aging firm Fountain Life.
Celularity stock is down more than 90% in the past 12 months.

Athersys
Athersys’s MultiStem bone marrow product is sometimes mixed up with MSCs, but the firm describes it this way, “MultiStem clinical product is developed from a special class of stem cells called Multipotent Adult Progenitor Cells”. I think of them as cousins of MSCs.
MultiStem is in trials for ARDS, trauma, and stroke. So far the data from trials have not been super encouraging.
Athersys is another firm that jumped into the COVID space during the heart of the pandemic.
The stock is down 77% in the past year and more than 99% over the past five years. Its future is unclear.

Mesoblast
Mesoblast is an Australian, MSC-focused biotech. Unlike the other biotechs in today’s post, they have an approved cellular therapy even if that approval came outside the U.S. So that distinguishes them from many others.
Their remestemcel-L product appears to provide some benefit for pediatric GvHD.
They’ve been dancing with the FDA for a long time about getting approval in the U.S. Most recently the FDA asked for a new trial for remestemcel-L, which was bad news.
The stock is down more than 50% in the past year. It’s down more than 80% in the past 5 years.
Still, I think there could be something positive there for specific cases of GvHD. Is that alone a tenable target for a biotech? They’ve also been exploring other diseases.

BrainStorm Cell Therapeutics
BrainStorm has been trialing its product Nurown for ALS. The trial results have generally been discouraging. However, the firm argues that in subgroups of ALS patients there may be a meaningful benefit.
An FDA advisory panel recently voted against Nurown moving forward.
The stock is down more than 95% in the past 12 months.
Lessons looking ahead
What does this tetrad of bad news tell us about stem cell stocks and biotechs more generally? One thing we already knew — this is an extremely risky sector. Cell therapy development may be more challenging than pharmaceutical drug development.
It’s also super risky to have a cell therapy product in search of a disease to treat with it, which has happened in some cases in the cell therapy space.
There are other cell therapy and regenerative medicine stocks are doing much better. I’m going to do a follow-up post on at least four of those that have brighter prospects.
Note that this post is not investing advice.
Hi Paul,
I’ve been watching Mesoblast quite closely over the past 10 years; it’s been an interesting journey watching them try to commercialize their research/products.
The share price has gone from ~$0.70c, up to $7.00 or so, and now all the way back down to $0.30c. For investors, this is a wild ride, luckily I am not an investor – but more so spectating from the side line!
However for stem cell enthusiasts like myself, it’s a positive thing – as I see Mesoblast as somewhat of a pioneer in trying to get the world to see stem cells in a different light and pushing hard to commercialize regenerative use-cases.
In my personal opinion, more needs to be done outside of the scientific community to dispel myths on stem cells and explain it in simple language. With greater understanding, comes greater societal push and understanding of what the benefits of stem cell research and related medicine can do.
I created https://www.stemcellhelper.com/ for this very reason.
Would love to hear your professional opinion on how we can better educate the broader world on stem cells, given it could help unlock new ideas/regulatory bodies to increase interest, etc.
Regards,
Alex
Hi Paul,
Yeh it’s been a complete and total disaster. As you know, I’ve been following many of these stocks for a long time. I have been overly optimistic and taken my licks. Avita Medical has been an exception to these failures and is commercializing a device that produces an autologous cocktail of various regenerative skin cell types. I wonder if you would characterize Avita (RCEL) as a stem cell stock?
Hi WST,
I wish things had turned out differently. I’m going to read up on Anita Medical. Sounds interesting.
If I may, Paul?…In support of funding Athersys, and their “MASTERS-2” pivotal phase 3 clinical trial for Acute Ischemic Stroke patients…My (2) “Public Comments” sent to CIRM (California Institute for Regenerative Medicine) for their recent “August 25 (2023)Task Force on Neuroscience and Medicine Meeting” -https://www.cirm.ca.gov/agendas/august-25-task-force-on-neuroscience-and-medicine-meeting/ (As listed under “Open Session”, 5. Public comment –
1st “Letter to the Board” – https://www.cirm.ca.gov/wp-content/uploads/2023/09/825-Neuro-Task-Force-Public-Comment.pdf
2nd “Letter to the Board” – https://www.cirm.ca.gov/wp-content/uploads/2023/09/825-Neuro-Task-Force-2nd-Public-Comment.pdf
Thank You, Paul!
PS. Maybe things are beginning to change in a positive way for Athersys?…See this recent 10/3/2023 PR, “Athersys Licenses its Animal Health Assets to Ardent Animal Health” – https://www.athersys.com/investors/press-releases/press-release-details/2023/Athersys-Licenses-its-Animal-Health-Assets-to-Ardent-Animal-Health/default.aspx
John Redaelli
Lack of mechanism of action appeared to be the biggest deal…along with BrainStorm’s post-hoc analysis of a failed trial that sought to single out characteristics of those who seemed to improve. But there wasn’t much to work with.
Dear Admin:
I hope the tech development leaders of each of these companies, and all others like them conducting “stem cell trials” with cell preparations of unknown tissue stem cell dosage, are reading The Niche and its comments like the one I am leaving now.
If tissue stem cells are the essential ingredient for their treatments, how can they ever succeed in demonstrating efficacy, if they have no idea of how many tissue stem cells they are administering in treatments? They are all using tissue cell preparations that are cell heterogeneous (i.e., containing stem cells, lineage-committed progenitor cells, arrested differentiating cells), in which the stem cells are often a small sub-fraction. In the case of expanded “stem cell” products, there are likely to be no tissue stem cells present at all. In this regard, most stem cell medicine is the most ridiculous medicine practiced, because it completely ignores one of the most fundamental principles of medicine, which is that dose matters.
James @ Asymmetrex®
Most clinical trials, and nearly all approved cell therapies, have gone through an incredibly rigorous QC qualification with dedicated testing requirements for end products. Cell dosage and cellular composition is usually highly quantified by flow cytometry and other other high complexity tests. These tests are often required by FDA and part of the CLIA testing paradigm. Ones that have little to no product characterization don’t get FDA clearance, and rarely get very far.
Expansion technologies vary, and depending on the stem cell source can be highly effective. Look at GamidaCell’s Omisirge product that gained FDA approval earlier this year. Full potency of expanded HSC’s, highly characterized, and far better than any other HSC source/product out there.
Dear STEMCELLSCIGUY:
Thanks for being what is surely an unwitting foil for my continuing education on this issue. NONE of the “incredibly rigorous QC qualification with dedicated testing requirements for end products” distinguishes stem cells from progenitor cells. The FDA’s Standards Coordinating Body (SCB) currently lists this ability as a needed standard for regenerative medicine clinical trials. The relative fraction of these two cell subtypes present in all human tissue stem cell-containing preparations, whether fresh or cultured, is a critical determinant of transplantation treatment outcomes, whether in approved therapies or in clinical trial treatments. Categorically, there are NO biomarkers, or combination of biomarkers, that make this distinction by flow cytometry. In the case of HSCs, for the past two decades, the only method available was the SCID mouse repopulating cell assay; and for other tissue stem cell types, like mesenchymal stem cells, there has been NOTHING.
You should read the GamidaCell updated clinical trial reports more closely. Long ago, GamidaCell correctly stopped claiming to be expanding “HSCs;” because even if they were, they had not reported it for cumbersome SCID mouse repopulating cell assay data, which might have indicated that they were. Despite being often misquoted, their recent clinical trials show efficacy for short-term clinical outcomes, not long-term engraftment. Expanded progenitors alone will give then their current results. Long-term engraftment can be improved by progenitors, but sufficient HSC dosage is obligatory. Importantly, their successful trials, so far, have not been designed to evaluate long-term engraftment outcomes. They have been quite responsible in their written claims, but others often conflate their success with HSC expansion, which in fact was not reported.
James @ Asymmetrex®
James,
It is fine to quibble over semantics and minute details. Facts are that we do not have the ability to fully characterize long term or ‘Master’ HSC’s. All medical science currently can do is use the tools they currently have, and work with incredible engraftment data that GamidaCell has shown.
No one can deny that the FDA was very hard on GamidaCell, which nearly broke the company. As the FDA is pretty much cited as the ‘most rigorous and demanding’ of any worldwide competent authority, all the things required of them to achieve an approved product more than meets any logical Quality requirements. Would more be better? Would a fully functional DNA/RNA map of ‘master level’ HSC be ‘the best’? Sure? But since it does not exist, and since finding it might require massive use of developing embryos, or sacrifice of human life…. it may be a long time coming.
Obviously, there will be follow-up of long-term engraftment. Once we get 20, 30, 40, 50 years it will be interesting to see. Medical science normally does not wait that long for any indication under circumstances like (various) cancer, in terms of long-term clinical trials. If we only had a time machine. But, as published in Blood, there is earlier clinical work with the product that shows “…long-term follow-up from the earlier studies of omidubicel6 suggest robust, durable engraftment for more than 10 years, confirming the persistence and possible expansion of long-term repopulating cells during the ex vivo culture period”
And on the point of the term ‘Stem Cell,’ that too is just a word, a construct. The term ‘stem cell’ has been applied across a wide swarth of cells, from embryonic to adult cells. We use terms like progenitor freely too. But all those definitions are incredibly blurry, and science uses them generally, as their full meaning is somewhat murky depending on who you ask… MSCs anyone? So again, more characterization is always the goal, but medicine would stagnate if we had to wait 30 years for a worldwide consensus on what a ‘true’ stem cell was.
It is appreciated for the push for better science. Its most everyone’s goal. One must note however that your interests do come with the disclaimer that your company sells tests for counting ‘stem cells’ with a novel and proprietary method. In addition, Asymmetrex sells ‘expanded stem cells’ on its site as well. We all appreciate you wear your company name on your tag, but it does raise the shadow of some conflict of interest. Do your posts push for better cell characterization, or the use of your technology? Hopefully you would say ‘both’, but therein lies a conflict.
I’d like to draw attention to the recent meeting between BrainStorm and the FDA. It’s available on YouTube, and it would be great content for a 1-day training session on understanding interactions with the FDA.
https://www.fda.gov/advisory-committees/advisory-committee-calendar/cellular-tissue-and-gene-therapies-advisory-committee-september-27-2023-meeting-announcement
I only watched part of it but it was really interesting. The panel focused a lot on the rationale and how clinical data should be analyzed as well as potential for harm.